JSTORIES ー 蛀牙或是年齡增長所導致的缺牙,傳統上都是以假牙或植牙等人工材料來取代,但目前正在研發的一種新藥,將可為醫學治療鋪路,創造出 「第三顆牙齒」,以取代未來失去的恆齒。
全球首創的牙齒再生治療藥正由總部位於京都市的 TreTjem BioPharma(トレジェムバイオファーマ) 開發。該公司目標是在 2024 年 9 月開始進行臨床試驗以確認該藥物的安全性後,於 2030 年將其商品化,隨後針對先天性部分牙齒缺失的 「先天性無齒症」 患者進行治療試驗,目標是在 2030 年之前將該藥物投入實際使用。
目前,這種再生藥物針對的是先天性無牙症患者,這種罕見的疾病是指一個人出生時就沒有牙齒或只有少量牙齒。 估計總人口中有 1% 的人患有此症,假牙和植牙是目前最常見的治療方式。但由於兒童較不適宜植牙,多數人得忍受生活的不便。未來,這項治療有望擴展至高齡者以及因蛀牙等原因缺牙的人,造福更多患者。
該藥物由專攻口腔外科的 高橋先生 等人在京都大學研究生院開發。目前隸屬於 公益財團法人田附興風會醫學研究所北野醫院(大阪市) 的高橋先生,自約30年前起便開始探索牙齒再生的可能性。
開發的轉折點出現在2018年。最初,高橋先生等人嘗試利用基因改造病毒來促進牙齒生長,但始終未能取得顯著成果。隨後,他們將目光轉向一種名為 「USAG-1」 的蛋白質,這成為研究的重要突破口。
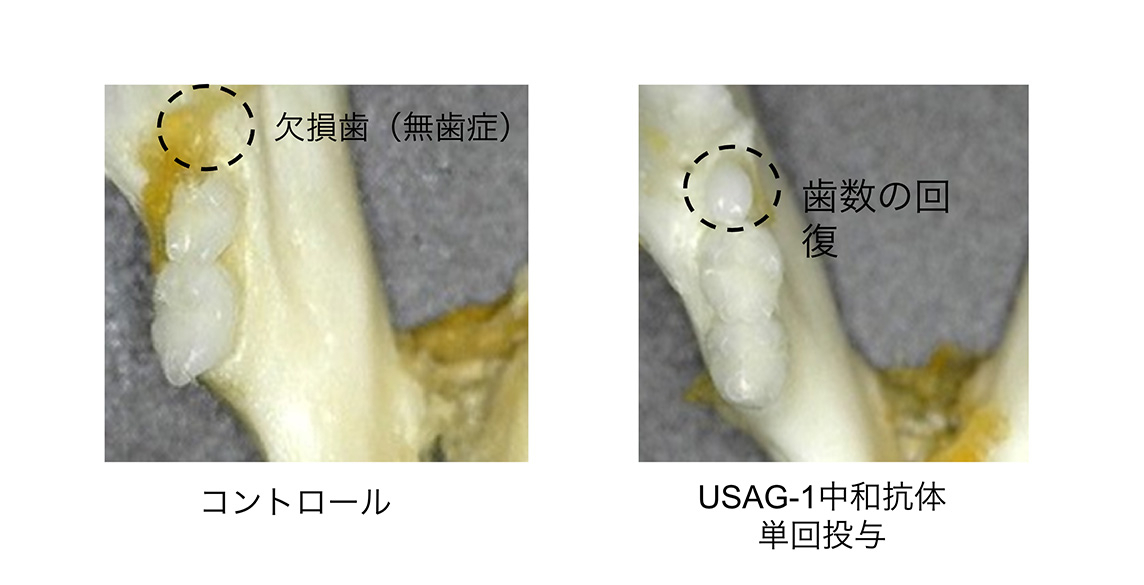
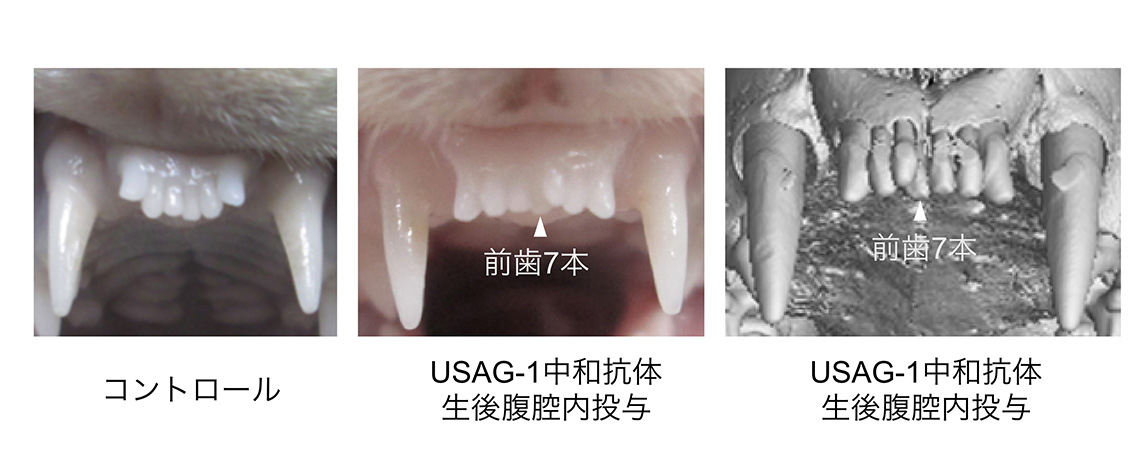
USAG-1 基因缺失的小鼠,其通常會退化消失的「牙胚」並未發生退化。高橋先生等人發現了這一現象,並通過投予抑制 USAG-1 蛋白質功能的抗體,成功實現了促進牙齒生長的實驗。
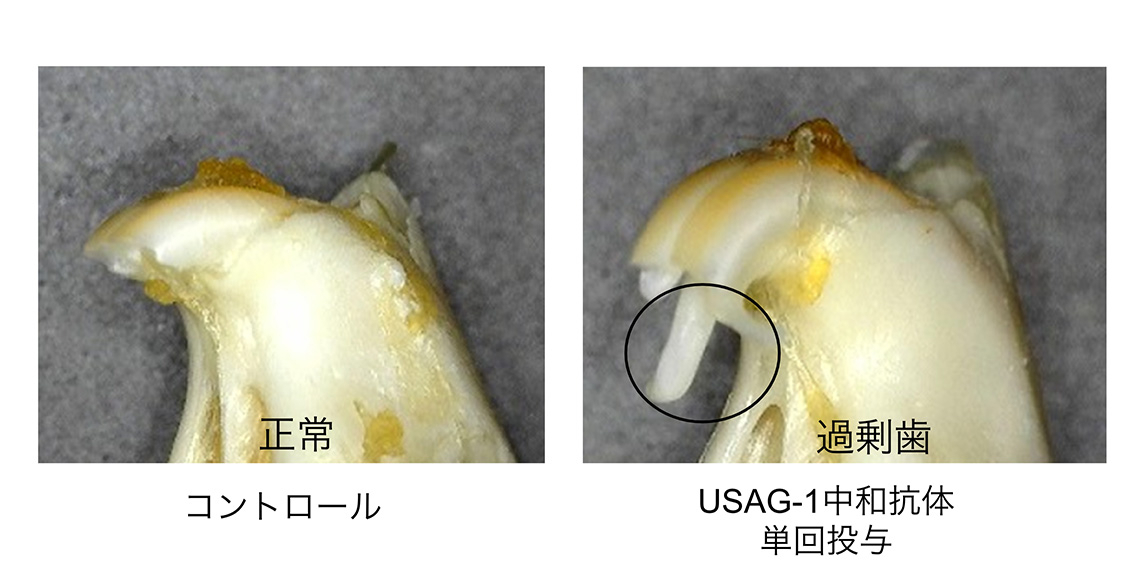
「沒有 USAG-1 蛋白質,抗體就無法形成。而製造這種蛋白質非常困難,」高橋先生表示。蛋白質的開發工作由 大阪大學蛋白質研究所 的 高木淳一教授 負責完成。
2020年5月,高橋先生等人創立了 TreTjem BioPharma(トレジェムバイオファーマ),並由曾在京都大學研究生院高橋研究室工作的牙醫 喜早ほのか 擔任代表。目前,該項目由來自京都大學、福井大學以及多家企業的100多人團隊共同推進開發工作。
目前,進入臨床試驗前的準備階段已基本完成。計劃從2024年9月起,用一年時間針對部分缺牙的30至65歲健康男性進行第一階段臨床試驗,以測試藥物的效果。隨後,將針對2至7歲無齒症患者進行藥物投與和效果驗證,目標在2030年內實現實用化。
該公司於2023年7月通過第三方定向增資籌集了總額3.8億日元的資金,用於臨床試驗藥物的製造等用途。為了推進藥物的實用化,該公司正積極尋求與製藥企業合作的可能性。
高橋先生表示:「牙齒是一個器官,而能夠再生出完整形態的器官,一直是我們的夢想。我希望這項技術能促進無齒症患者,特別是成長期兒童的營養攝取,並改善他們的生活品質。」
文章作者: 國分瑠衣子
編輯: 北松克朗
更新文章作者: 肱黑勇正
更新編輯: 一色崇典
首頁照片: TreTjem BioPharma 提供

_smallthumbnail.jpg)
_smallthumbnail.jpg)








![[PODCAST] 日本新科技助攻不孕症治療(Part4)](https://storage.googleapis.com/jstories-cms.appspot.com/images/1768443226894unnamed-5_bigthumbnail.jpg)







![[PODCAST] 如何打造成功的新創企業社群(第2集)](https://storage.googleapis.com/jstories-cms.appspot.com/images/1748493203370business-man-holding-light-bulb-social-network-2024-10-31-22-37-36-utc_smallthumbnail.jpg)


is this for all cases not just congenital because the fact is that millions of people are missing teeth and this would be better because it is natural...閲讀更多
I dearly hope that you find nothing but success & that it'll be so safe for people with health issues. I was diagnosed lupus when I was 14. I was hosp...閲讀更多
Dear honorable persons, My name is Daniel and I was born with the misfortune of growing up poor in the city of Las Vegas Nevada, USA. I have ...閲讀更多
Im sure a black market copy drug be available by your local street gang 2026.
God, how I wish they could do it. I'm 17, but I already have some problems with my teeth. I'm also often prone to caries. That's why I dream of restor...閲讀更多